German government buys large stock of newly approved Covid medicine
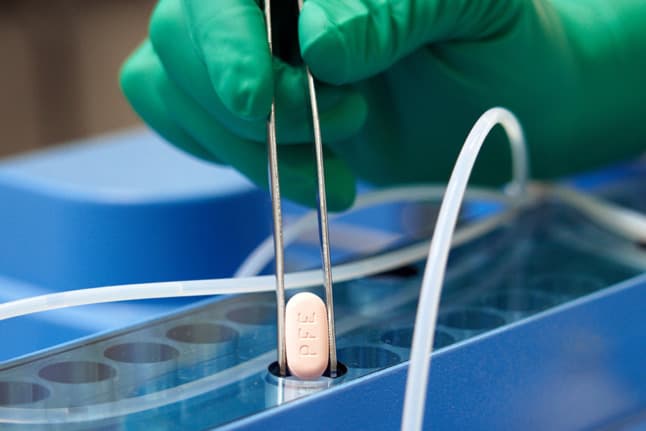
Germany has ordered one million packets of a new Covid medicine designed to prevent severe illness and hospitalisation, Health Minister Karl Lauterbach (SPD) announced on Tuesday.
The drug, which is manufactured by Pfizer under the brand name Paxlovid, is the first anti-Covid medication that can be taken in tablet form and reportedly offers protection against severe courses of Covid.
"The drug is extremely promising because it can significantly weaken the severe course of Covid when administered early," Health Minister Karl Lauterbach told DPA on Tuesday. "I expect that we will be able to prevent numerous severe courses in intensive care units with it."
The United States' Food and Drug Administration (FDA) issued an emergency approval for the new drug just before Christmas, giving doctors an additional treatment option in the fight against severe Covid.
READ ALSO: German health agency raises Covid risk level for the vaccinated and recovered
Lauterbach said Germany would be following suit with a similar emergency approval so that the drug could be used as soon as it is delivered in January.
"Slowly, through a combination of increasingly effective vaccines and treatment options, Covid is becoming a disease that will lose its terror," he said, adding that there would be cooperation with all drug manufacturers who were developing appropriate medicines to protect against hospitalisation or death from Covid.
"I'd rather that with rapid vaccination and effective drugs, we fight this battle than have to close schools," he added.
According to the manufacturer, patients are required to take three tablets twice a day for five days. All tablets are in one pack, which corresponds to one treatment cycle.
Paxlovid consists, among other things, of the active ingredient nirmatrelvir, which inhibits a Sars-CoV-2 protein in order to stop the multiplication of the virus.
The FDA has recommended that the drug is used to treat positive Covid patients aged 12 and older with mild to moderate symptoms and a high risk of a worsening disease. The European Medicines Agency (EMA) has also taken a view on the new medicine, saying that Paxlovid could be used to treat adult patients who do not need supplemental oxygen but who are at increased risk for a severe course of Covid.
The drug has not yet been officially approved in the EU, but testing is ongoing.
89 percent protection
According to Pfizer, the pills have been very successful in preventing severe disease progression in high-risk patients: an interim analysis of test results showed that the drug reduced the risk of hospitalisation and death in Covid-19 patients by 89 percent.
Possible side effects include impaired sense of taste, diarrhoea, high blood pressure and muscle pain. The EMA had also said that the drug shouldn't be taken by patients with severe kidney or liver dysfunction and should not be taken in combination with certain other medicines because of drug interactions. The drug is not recommended for pregnant women.
Drugs like Paxlovid are considered by experts to be a pillar in the fight against the coronavirus, though compared to preventive vaccinations, they are significantly more expensive and often more complicated to use.
In its emergency approval this month, the FDA stated that the drug was not a substitute for vaccination for the general population.
READ ALSO: German Ethics Council recommends extending vaccine mandates
Comments (2)
See Also
The drug, which is manufactured by Pfizer under the brand name Paxlovid, is the first anti-Covid medication that can be taken in tablet form and reportedly offers protection against severe courses of Covid.
"The drug is extremely promising because it can significantly weaken the severe course of Covid when administered early," Health Minister Karl Lauterbach told DPA on Tuesday. "I expect that we will be able to prevent numerous severe courses in intensive care units with it."
The United States' Food and Drug Administration (FDA) issued an emergency approval for the new drug just before Christmas, giving doctors an additional treatment option in the fight against severe Covid.
READ ALSO: German health agency raises Covid risk level for the vaccinated and recovered
Lauterbach said Germany would be following suit with a similar emergency approval so that the drug could be used as soon as it is delivered in January.
"Slowly, through a combination of increasingly effective vaccines and treatment options, Covid is becoming a disease that will lose its terror," he said, adding that there would be cooperation with all drug manufacturers who were developing appropriate medicines to protect against hospitalisation or death from Covid.
"I'd rather that with rapid vaccination and effective drugs, we fight this battle than have to close schools," he added.
According to the manufacturer, patients are required to take three tablets twice a day for five days. All tablets are in one pack, which corresponds to one treatment cycle.
Paxlovid consists, among other things, of the active ingredient nirmatrelvir, which inhibits a Sars-CoV-2 protein in order to stop the multiplication of the virus.
The FDA has recommended that the drug is used to treat positive Covid patients aged 12 and older with mild to moderate symptoms and a high risk of a worsening disease. The European Medicines Agency (EMA) has also taken a view on the new medicine, saying that Paxlovid could be used to treat adult patients who do not need supplemental oxygen but who are at increased risk for a severe course of Covid.
The drug has not yet been officially approved in the EU, but testing is ongoing.
89 percent protection
According to Pfizer, the pills have been very successful in preventing severe disease progression in high-risk patients: an interim analysis of test results showed that the drug reduced the risk of hospitalisation and death in Covid-19 patients by 89 percent.
Possible side effects include impaired sense of taste, diarrhoea, high blood pressure and muscle pain. The EMA had also said that the drug shouldn't be taken by patients with severe kidney or liver dysfunction and should not be taken in combination with certain other medicines because of drug interactions. The drug is not recommended for pregnant women.
Drugs like Paxlovid are considered by experts to be a pillar in the fight against the coronavirus, though compared to preventive vaccinations, they are significantly more expensive and often more complicated to use.
In its emergency approval this month, the FDA stated that the drug was not a substitute for vaccination for the general population.
READ ALSO: German Ethics Council recommends extending vaccine mandates
Join the conversation in our comments section below. Share your own views and experience and if you have a question or suggestion for our journalists then email us at [email protected].
Please keep comments civil, constructive and on topic – and make sure to read our terms of use before getting involved.
Please log in here to leave a comment.