German pediatric heart device receives FDA okay
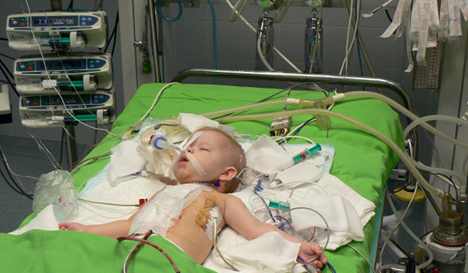
The US Food and Drug Administration has approved a first mechanical cardiac assist device for children that can help keep patients alive as they await a transplant.
The EXCOR Pediatric System, made by the German company Berlin Heart, can be sized to fit young people from newborns to teenagers.
"This is a step forward. It is the first FDA-approved pulsatile mechanical circulatory support device specifically designed for children," said Susan Cummins, chief pediatric medical officer in the FDA's Center for Devices and Radiological Health.
"Previous adult heart assist devices were too large to be used in critically ill children to keep them alive while they wait to get a new heart," she added in a statement Friday.
The device was tested on a group of 48 US patients, and was found to improve survival rates for transplant patients compared with the use of extracorporeal membrane oxygenation, the current standard of care, though not FDA approved.
As a whole, a reported 12-17 percent of children and 23 percent of infants die while they await heart transplants, according to the FDA.
AFP/mw
Comments
See Also
The EXCOR Pediatric System, made by the German company Berlin Heart, can be sized to fit young people from newborns to teenagers.
"This is a step forward. It is the first FDA-approved pulsatile mechanical circulatory support device specifically designed for children," said Susan Cummins, chief pediatric medical officer in the FDA's Center for Devices and Radiological Health.
"Previous adult heart assist devices were too large to be used in critically ill children to keep them alive while they wait to get a new heart," she added in a statement Friday.
The device was tested on a group of 48 US patients, and was found to improve survival rates for transplant patients compared with the use of extracorporeal membrane oxygenation, the current standard of care, though not FDA approved.
As a whole, a reported 12-17 percent of children and 23 percent of infants die while they await heart transplants, according to the FDA.
AFP/mw
Join the conversation in our comments section below. Share your own views and experience and if you have a question or suggestion for our journalists then email us at [email protected].
Please keep comments civil, constructive and on topic – and make sure to read our terms of use before getting involved.
Please log in here to leave a comment.